Ammonia (NH3) and Organic Nitrogen Destruction Catalysts
We developed ammonia destruction catalysts treating NH3 emitted from semiconductor manufacturing processes, wastewater, chemical plants, and so on.
When NH3-containing exhaust gas is combusted with conventional oxidation catalysts, the ammonia itself is destroyed. However, the generation of NOx and N2O as by-products is an issue. Our NH3 destruction catalysts can reduce dramatically the generation of NOx and N2O while additional NH3 is not required (high concentrations of about 1% can be handled without gas dilution). It can also similarly treat organic nitrogen compounds.
No harmful substances (such as vanadium pentoxide (V2O5)) are contained in our catalysts.
Targeted gases:
Ammonia, hydrogen cyanide, acrylonitrile, and other organic nitrogen compounds (DMF, NMP, trimethylamine, etc.)
Catalyst Specifications
| Catalyst type | IKOMAC・NHN series |
|---|---|
| Shape | Honeycomb monolith |
| Number of cells | 200 cells/inch2 |
| Standard size | 150×150×50t mm |
| Apparent bulk density | Approx. 0.7 g/cc |
| Allowable temperature range |
280℃~ |
Achievements
Stripper exhaust gas, excess ammonia, lithium-ion batteries, polyimide films, semiconductors, power plants, sewage treatment, chemical industry, agricultural chemicals, food industry, synthetic fibers, electrodeposition coating, and many others.
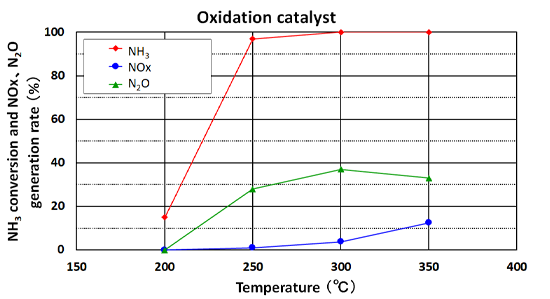
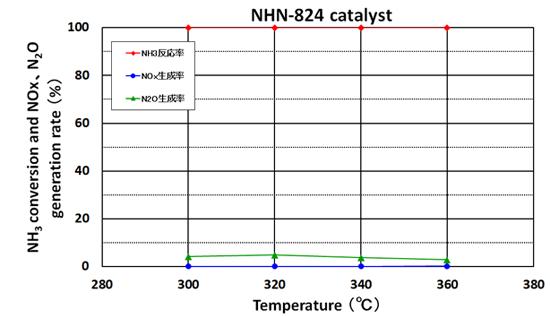
Ammonia Conversion
(comparison of oxidation catalysts and ammonia destruction catalysts)
When treating ammonia with conventional oxidation catalysts, large amounts of NOx and N2O are generated, requiring a secondary treatment (left figure). However, the ammonia destruction catalyst with both its oxidation and reduction functions can efficiently decompose ammonia and suppress the generation of NOx and N2O simultaneously (right figure).
For inquiries:
| Department | Environmental Catalyst Div. |
|---|---|
| Contact Form | |
| TEL | ARIOKA:+81-90-5398-5199 (Japanese, English) PIET:+81-80-4462-6530 (French, English) *Do not hesitate to reach out to us by using the contact form, we will come back to you as soon as possible. |
