NH3(Ammonia) Cracking Catalyst
- Currently, ammonia is getting attention as an energy carrier in the pursuit of a carbon-neutral society.
- We are focusing not only on leaked NH3 and N2O treatment catalysts when NH3 is burned as fuel, but also on NH3 cracking.
- When using NH3 as an energy carrier, it is not only used directly as fuel, but also in cases where a large portion of NH3 is cracking into H2 and used in generators, etc..
- In this case, the purity required for the NH3 is not so high.
- However, when H2 is required as fuel for applications such as a Polymer Electrolyte Fuel-Cells(PEFC), high-purity H2 is necessary. Additionally, high NH3 cracking efficiency is required in the catalyst.
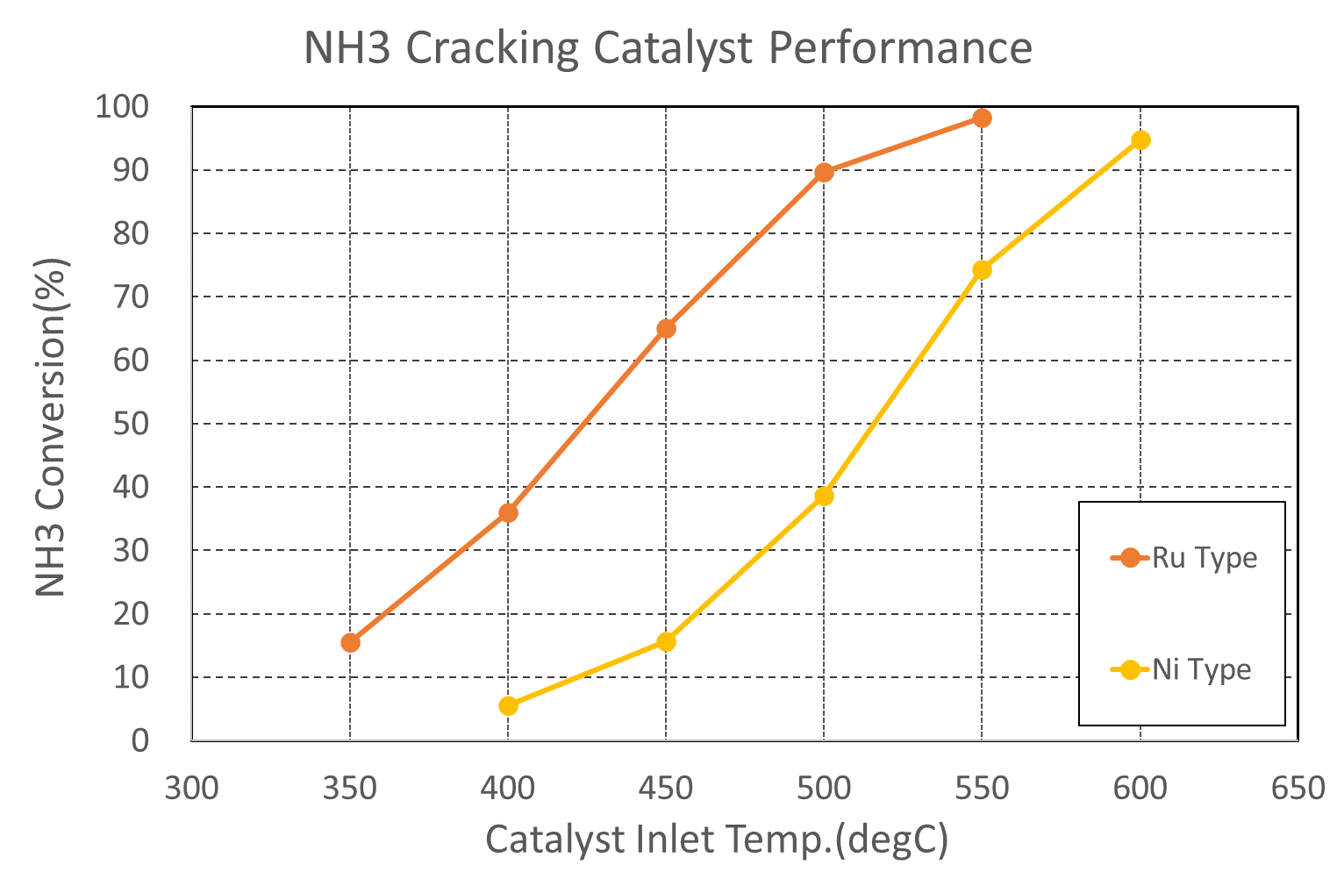
- We offer highly loaded Ni-based NH3 cracking catalysts and Low loaded Ru-based NH3 cracking catalysts that react from low temperatures in our lineup.
Reaction Mechanism
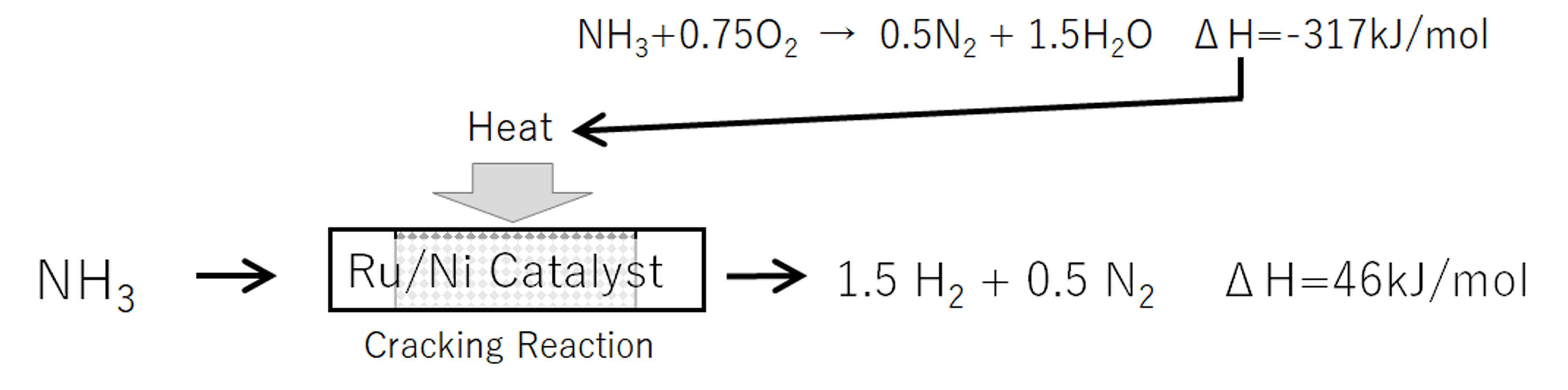
- The reaction of ammonia is the reverse reaction of NH3 synthesis as shown below. The cracking reaction proceeds easily at high temperatures and low pressure, but it is difficult in chemical equilibrium to completely cracking ammonia into hydrogen.
- Since the reaction of cracking ammonia to extract hydrogen is strongly endothermic, it requires appropriate heat supply.
- We have NH3 destruction catalysts that suppress the formation of NOx and N2O.
- By combining both catalysts and the combustion heat of NH3, we can offer a CO2-free heat input solution.
NH3 Cracking Catalyst Performance
Contact Sales Department
| Department |
Business Innovation Office |
|---|
| Contact Form |
|
|---|
| TEL |
81-3-5436-8484 |
|---|
| FAX |
81-3-5436-8680 |
|---|