CO₂ Adsorption and Desorption Material

| Model | NCA-03 |
|---|
A moisture-resistant CO₂ adsorption material that allows for carbon recycling.
Product Overview
This material adsorbs carbon dioxide (CO₂), a greenhouse gas that is a major contributor to global warming. The adsorbed CO₂ is then desorbed by heating the material, making it possible to achieve carbon recycling.
Features
- Humidity has little effect
- Desorption can take place at low temperatures
- High adsorption efficiency in low concentration
Specifications
Size and cell density can be chosen according to intended purpose.Also available in pellets (granule type).
| Type | Honeycomb, granules |
|---|---|
| Material | Inorganic metallic oxides |
| Size |
Compatible with any size. *Please specify the intended use for the product. |
What kind of gas is carbon dioxide (CO₂)?

Carbon dioxide is a colorless and odorless gas commonly referred to as CO₂. It may sound harmless, but CO₂ is a greenhouse gas that has the capacity to raise the Earth's surface temperature. The biggest cause of global warming in recent years is considered to be the increase in concentration of CO₂ in the atmosphere. Due to this, efforts to reduce CO₂ emissions are being made around the world.
Why has the concentration of CO₂ in the atmosphere increased?
Behind the increase in CO₂ is industrial development and contemporary affluent lifestyles. During the Industrial Revolution in the 18th century, fossil fuels such as coal, oil, and natural gas began to be used as energy sources. Burning these fossil fuels releases large amounts of CO₂ into the atmosphere, causing the concentration of CO₂ to increase every year. Furthermore, fossil fuels have become indispensible over time not only for industry, but also for people's daily lives. Gasoline and oil in particular have been used for a long time as fuel for cars and heating equipment, supporting the affluent lives of modern people. By using fossil fuels for energy, CO₂ emissions are increasing worldwide. At the same time, forests and wooded areas, which absorb CO₂ and contribute greatly to the prevention of global warming, are rapidly decreasing. This has become another major factor in the increase in CO₂.
Effects on the human body
CO₂ is always present in the air around us, and it is also emitted when humans and animals breathe. If large amounts of CO₂ accumulate in the air, this leads to an oxygen deficiency, which in turn can result in health hazards such as listed below.
Association between CO₂ concentration and discomfort and health hazards
| CO₂ concentration | Effects on living organisms |
|---|---|
| ~450 ppm | Typical atmospheric concentration |
| ~700 ppm | No particular health problems, even with prolonged exposure |
| ~1,000 ppm | No hazard to health, but some people may feel discomfort |
| ~2,000 ppm | Changes in physical condition become noticeable; many people will start to feel sleepy |
| ~3,000 ppm | One step away from a health hazard; some people experience stiff shoulders and headaches |
| 3,000 ppm~ | Symptoms such as headache and dizziness may occur, and prolonged exposure may be hazardous to health |
Is it true that CO₂ can be reused? What is carbon recycling?
The Japanese government has declared its goal of achieving carbon neutrality by 2050, and in addition to energy conservation, maximizing the use of renewable energy has become its policy. Carbon recycling is an initiative that captures and recovers CO₂ as a carbon resource and reuses it in chemicals, fuels, and minerals.
Mechanism for CO₂ adsorption
Zeolite is widely known as a general CO₂ adsorption material, but its efficiency decreases significantly when affected by moisture. Our company's CO₂ adsorption and desorption material is not easily affected by moisture, has high CO₂ adsorption performance even in environments with high humidity, and can efficiently recover CO₂ from the atmosphere (DAC: Direct Air Capture).
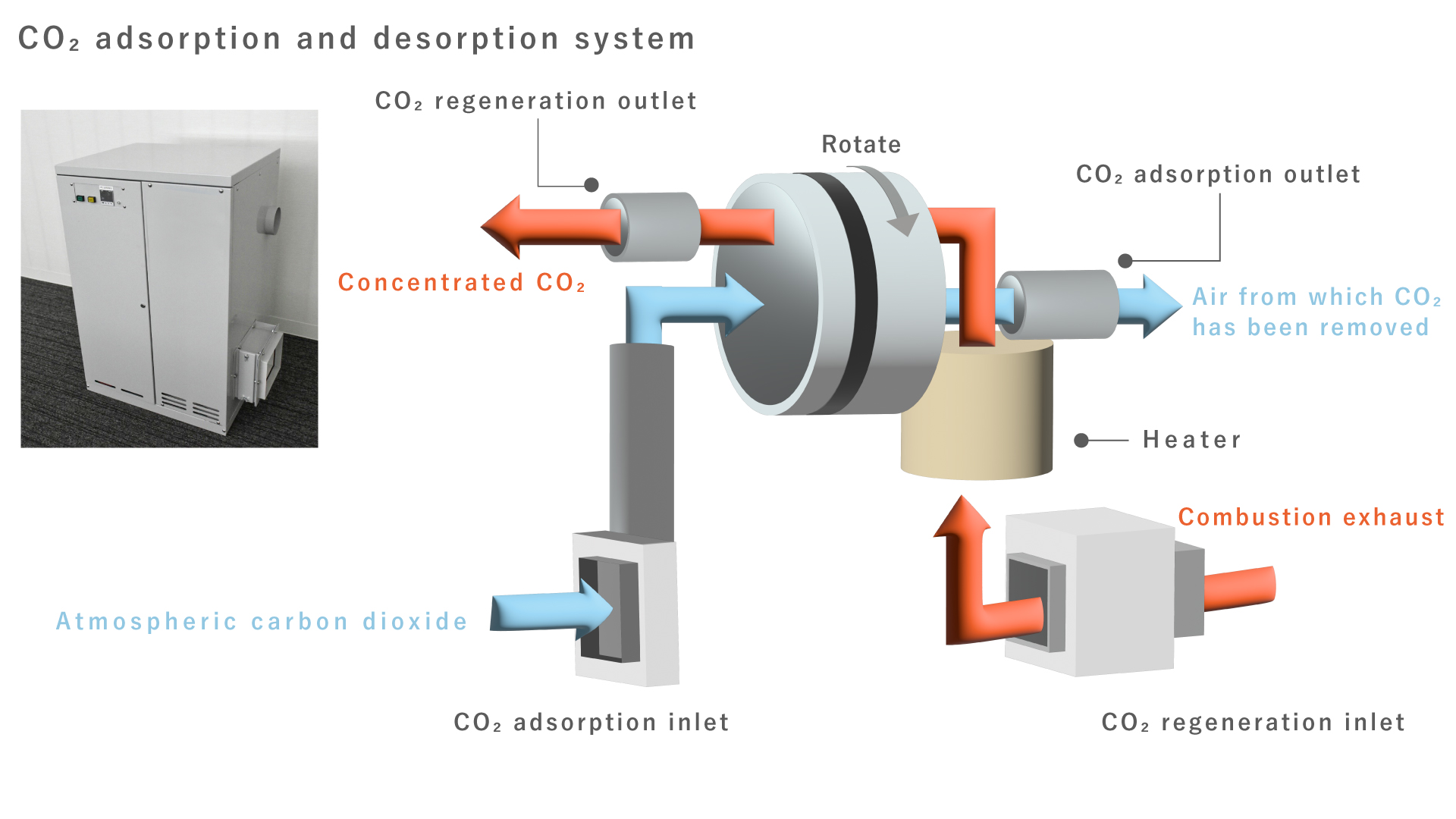
CO₂ adsorption and desorption system
Regarding CO₂ capture and utilization (CCU), as shown in the diagram below, after CO₂ in the atmosphere is adsorbed in the adsorption layer, the CO₂ is then desorbed from the CO₂ adsorption and desorption material by heating the desorption layer.
Contact Sales Department
| Department | Business Innovation Office |
|---|---|
| Contact Form | |
| TEL | 81-3-5436-8484 |
| FAX | 81-3-5436-8680 |
